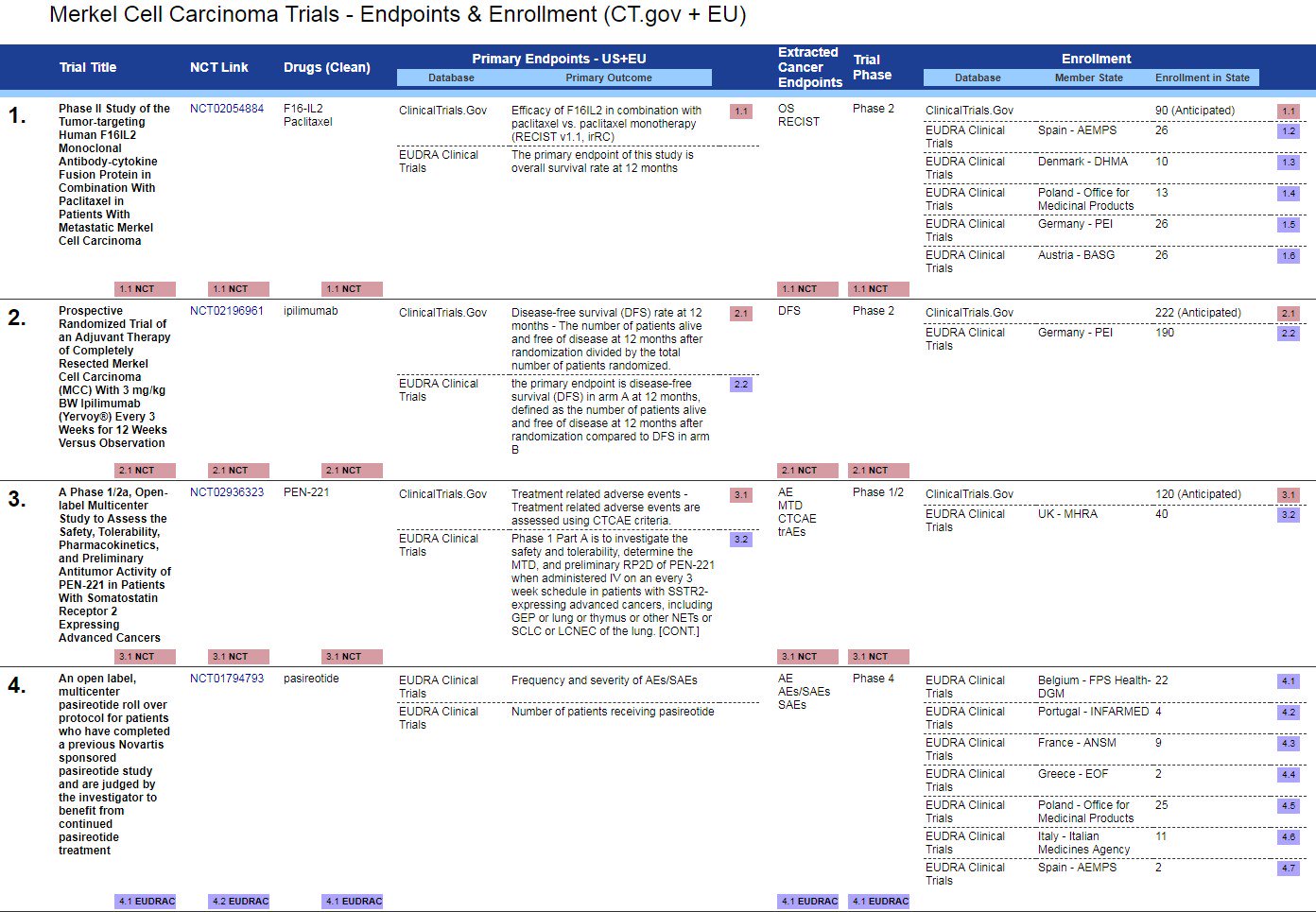
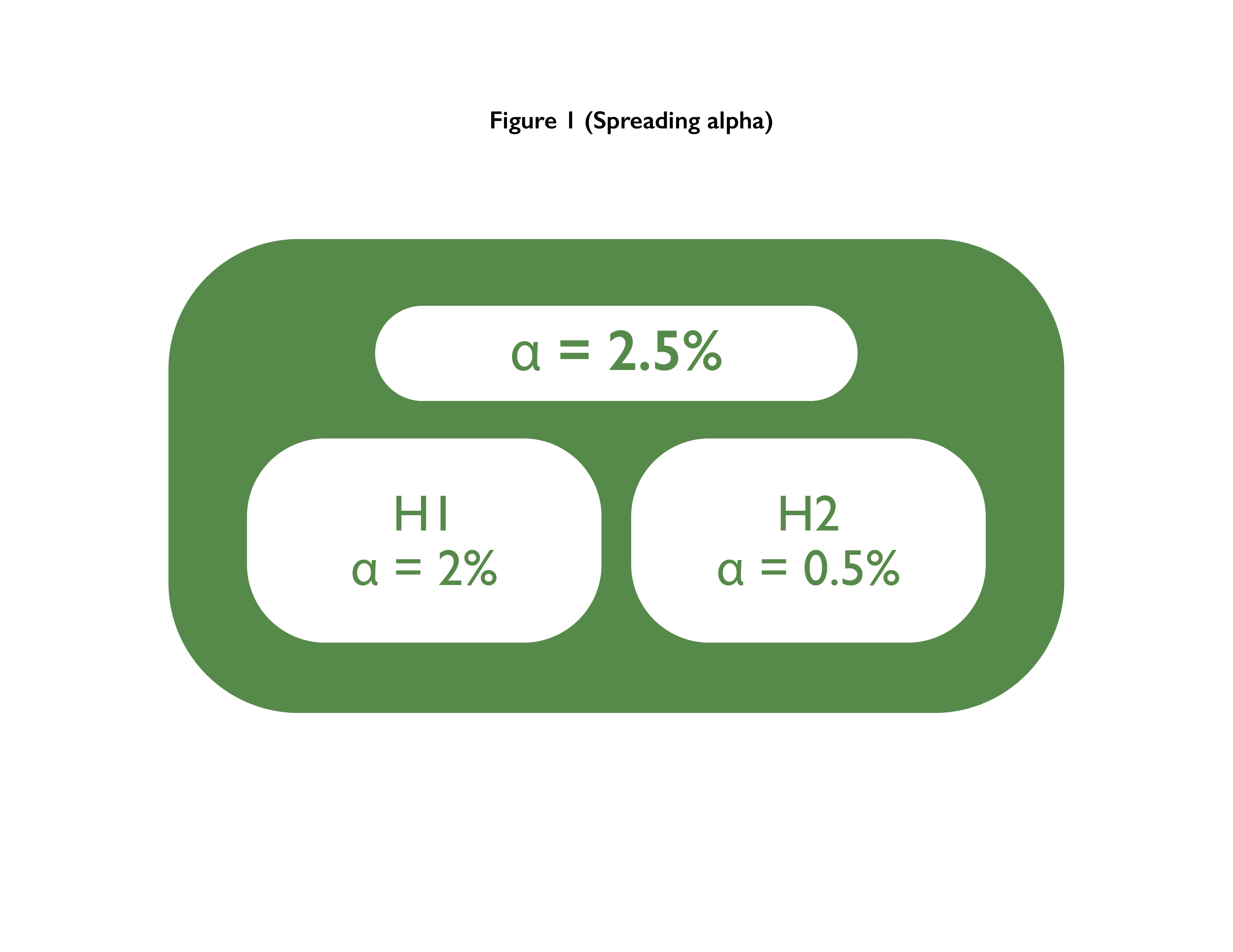
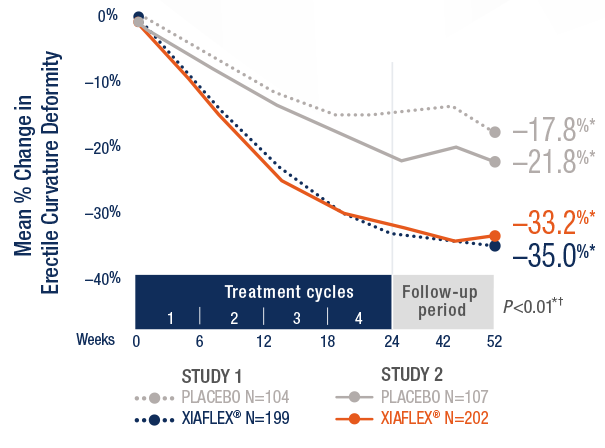
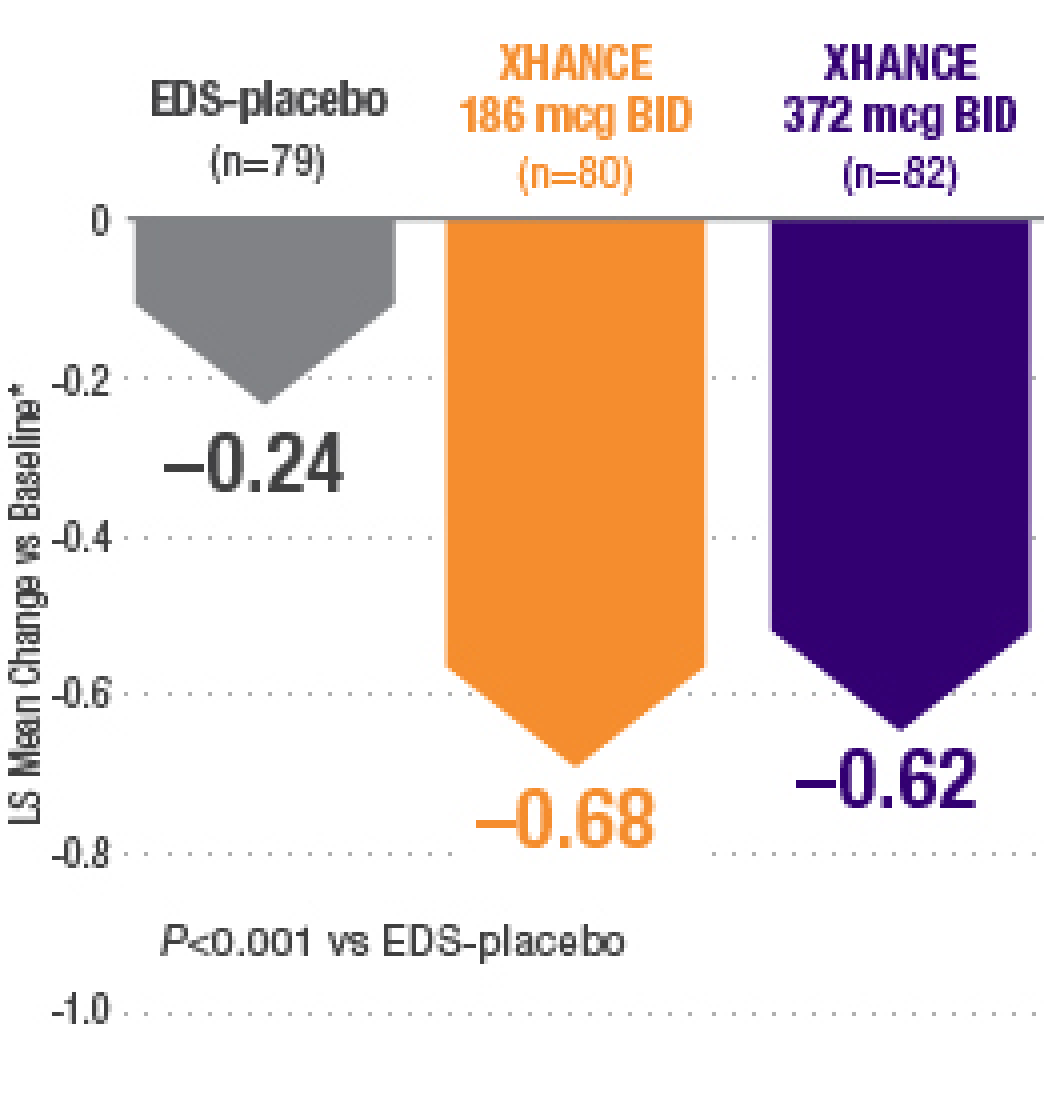
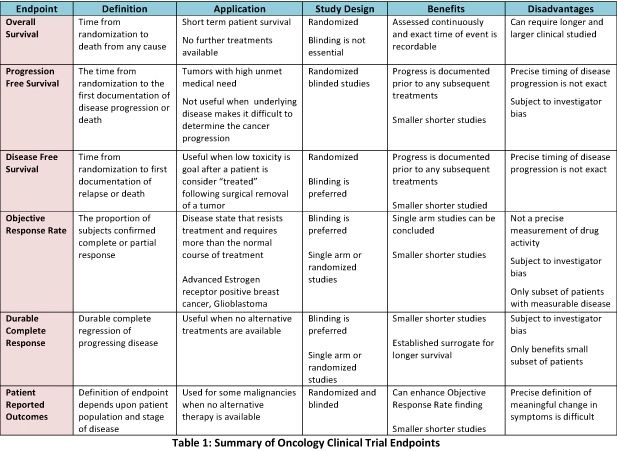
Multiple Co-primary Endpoints: Medical and Statistical Solutions A Report From the Multiple Endpoints Expert Team of the Pharmac
Multiple Co-primary Endpoints: Medical and Statistical Solutions A Report From the Multiple Endpoints Expert Team of the Pharmac

Metastases-directed Radiotherapy in Addition to Standard Systemic Therapy in Patients with Oligometastatic Breast Cancer: Study protocol for a randomized controlled multi-national and multi-center clinical trial (OLIGOMA) - Clinical and Translational ...

Pfizer Shares Co-Primary Endpoint Results from Post-Marketing Required Safety Study of XELJANZ® (tofacitinib) in Subjects with Rheumatoid Arthritis (RA) | Business Wire
Design, data monitoring, and analysis of clinical trials with co-primary endpoints: A review: Journal of Biopharmaceutical Statistics: Vol 28, No 1

Defining Endpoints and Biomarkers in Inflammatory Bowel Disease: Moving the Needle Through Clinical Trial Design - Gastroenterology
Sample size determination for a specific region in multiregional clinical trials with multiple co-primary endpoints | PLOS ONE
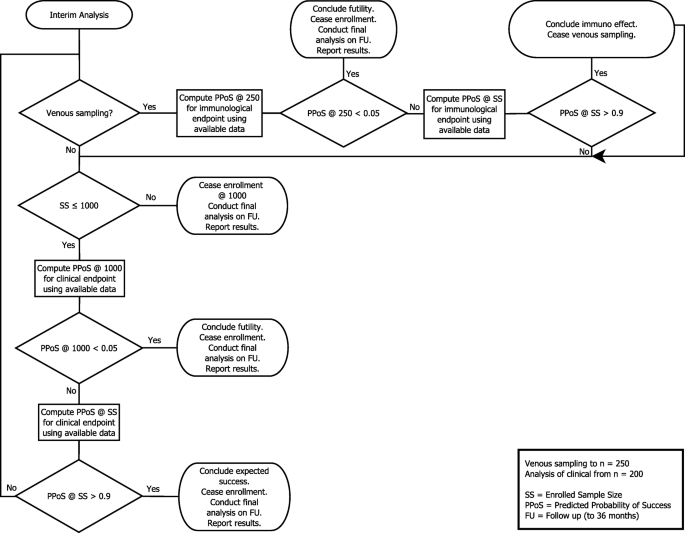
The ORVAC trial: a phase IV, double-blind, randomised, placebo-controlled clinical trial of a third scheduled dose of Rotarix rotavirus vaccine in Australian Indigenous infants to improve protection against gastroenteritis: a statistical analysis
Multiple Co-primary Endpoints: Medical and Statistical Solutions A Report From the Multiple Endpoints Expert Team of the Pharmac

Choice of Primary (or Co-primary) Endpoints: Efficacy, Safety, or Net Clinical Benefit in Superiority and Non-inferiority Trials | tctmd.com