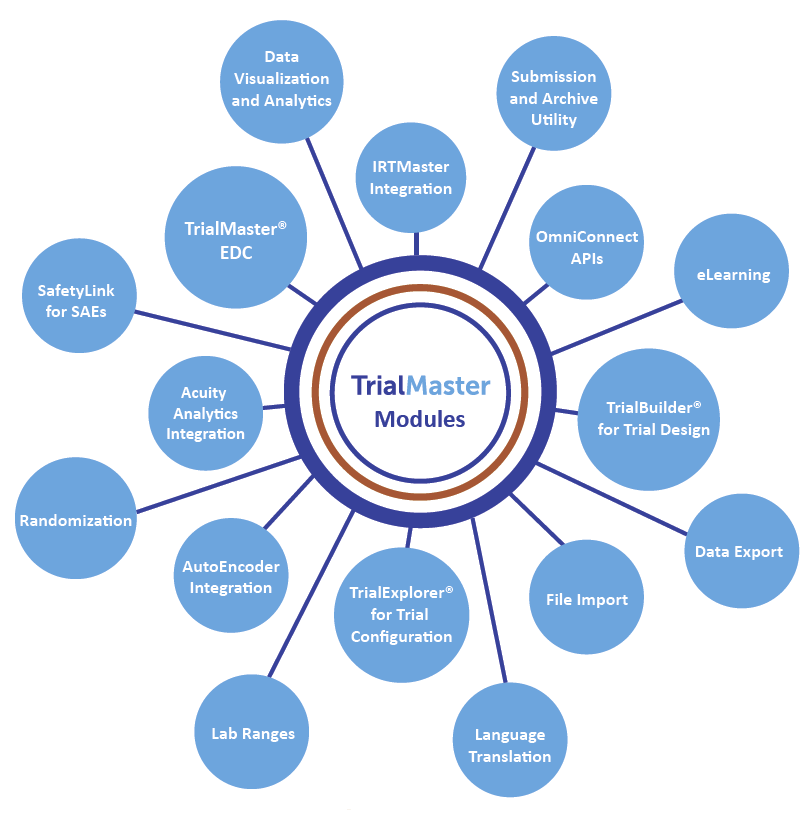
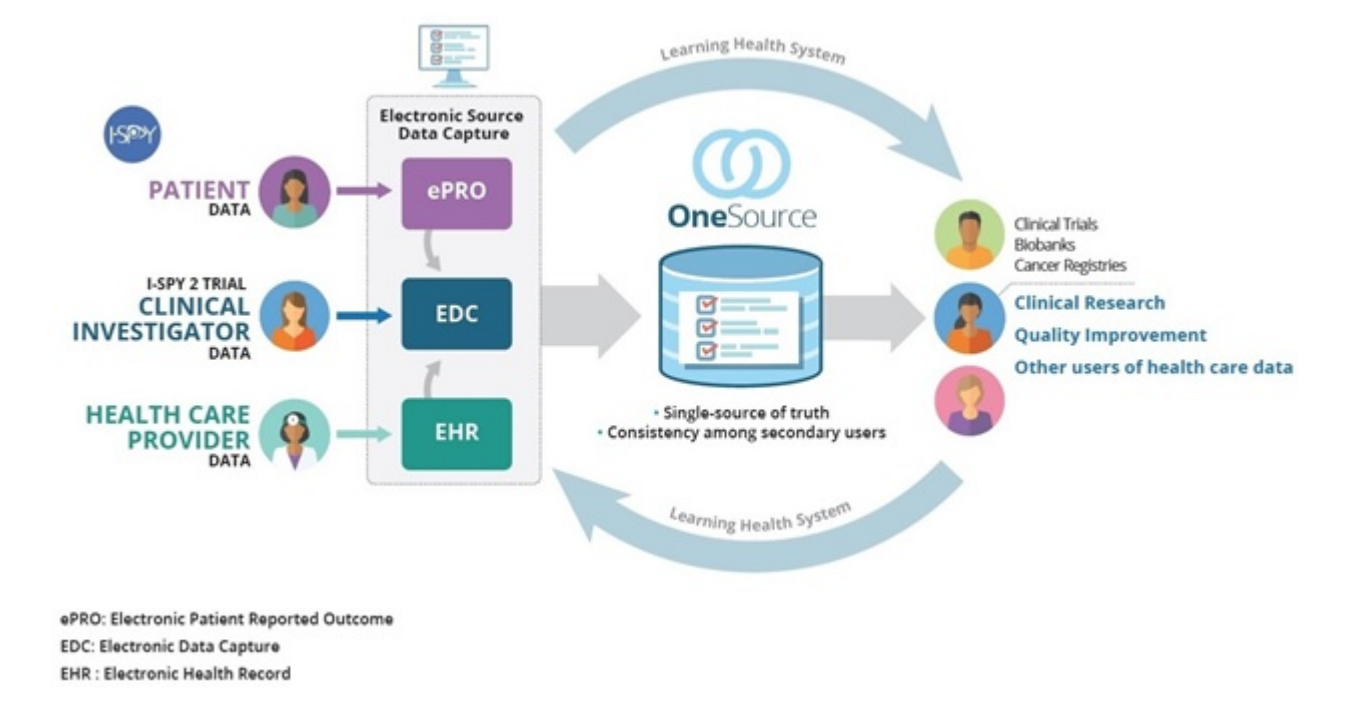
A schematic of data flow in a typical clinical study. eCRF, electronic... | Download Scientific Diagram

Top-rated electronic data capture system for clinical trials (Castor EDC) - with English subtitles - YouTube

End-to-end electronic data capture solution for clinical trial operations – Clinical trials and CRO centric systems
Electronic Data Capture Clinical Trials Ppt Powerpoint Presentation Inspiration Icon Cpb | Presentation Graphics | Presentation PowerPoint Example | Slide Templates

Pharma and biotecht Phase (I – II – III and IV) Clinical Trials | Electronic Data Capture (EDC) as a useful – ResearchManager

Clinical trials: Digital technology for recruitment, consent, and data capture | Hogan Lovells - JDSupra

Electronic Source (eSource) vs. Electronic Data Capture (EDC): What's the Difference? - Clinical Research IO - CRIO
![PDF] Electronic Data Capture in clinical trials– interface design and evaluation and system validation | Semantic Scholar PDF] Electronic Data Capture in clinical trials– interface design and evaluation and system validation | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/50a3193b5f8fb7f91479fd8839c1a6d03baba9b1/19-Figure2-1.png)
PDF] Electronic Data Capture in clinical trials– interface design and evaluation and system validation | Semantic Scholar





%2BDuring%2BClinical%2BTrials.jpg)