Commission Guideline — Guidance on posting and publication of result-related information on clinical trials in relation to the
Pharmaceuticals | Free Full-Text | Quality Assessment of Investigational Medicinal Products in COVID-19 Clinical Trials: One Year of Activity at the Clinical Trials Office | HTML
page 1 of 3 Version 1.0, 26 March 2020 Supplementary recommendations to the document European Guidance on the Management of Cl
May 2022 The rules governing medicinal products in the European Union VOLUME 10 - Guidance documents applying to clinical trials

PPT - REVISION OF EUDRALEX VOL. 4 - GMP Luisa Stoppa, Ph.D. Inspection and Certification Department PowerPoint Presentation - ID:6720277

Clinical trials were missing from regulatory documents of extended-release methylphenidate for ADHD in adults: a case study of public documents - Journal of Clinical Epidemiology
GUIDANCE DOCUMENTS CONTAINING THE COMMON PROVISIONS ON THE CONDUCT OF GCP INSPECTIONS BY COMPETENT AUTHORITIES OF THE DIFFERENT

Guidance for the Submission and Conduct of Clinical Trials (CT) with Medicinal Products - PDF Free Download
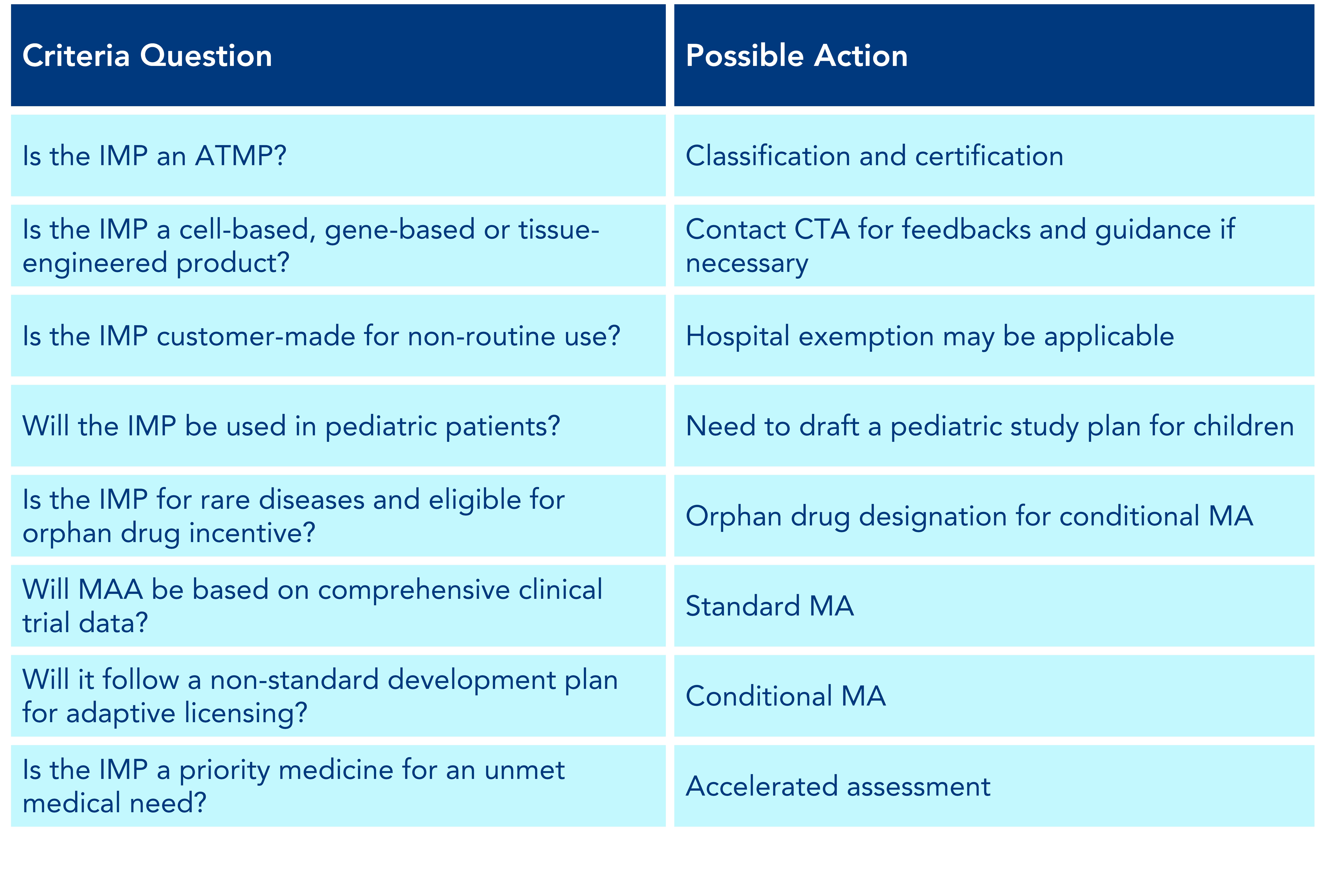
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience

PDF) Untangling the web of European regulations for the preparation of unlicensed radiopharmaceuticals | Clemens Decristoforo - Academia.edu