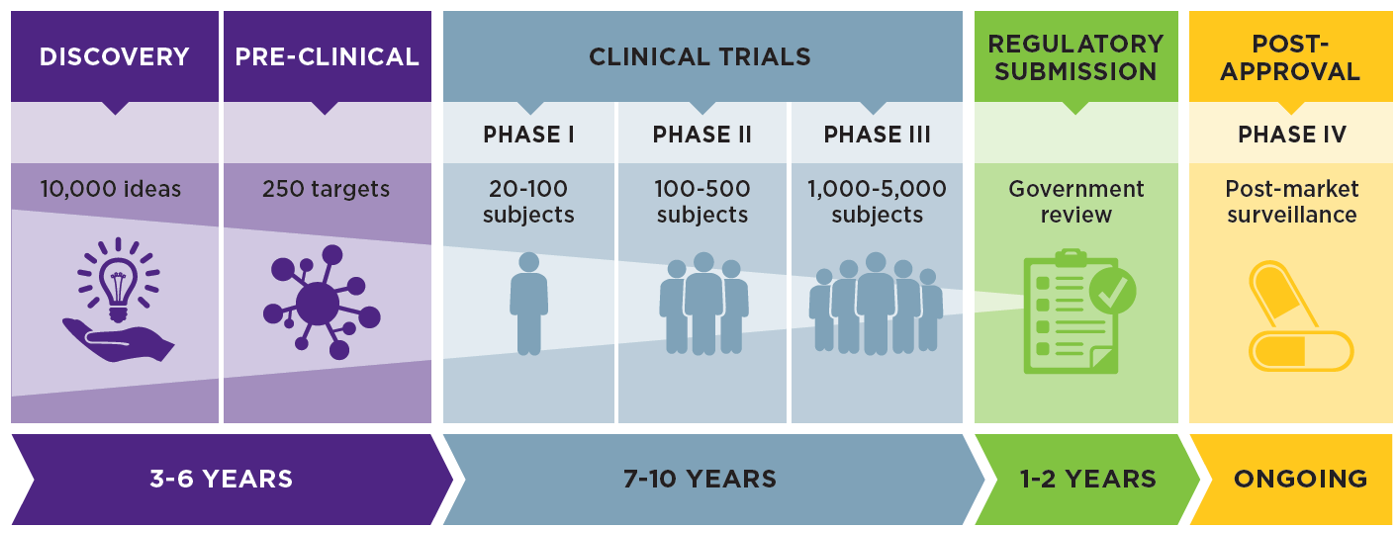
Avantor Clinical Services supports the need to fast-track clinical trials related to COVID-19 disease progression | Avantor
FDA Moves Against Fast Track Vaccines - News about Energy Storage, Batteries, Climate Change and the Environment
Cost-effectiveness of an integrated 'fast track' rehabilitation service for multi-trauma patients: A non-randomized clinical trial in the Netherlands | PLOS ONE

Breakthrough Drug is a Breath of Fresh Air for Cystic Fibrosis Patients — Journal of Young Investigators
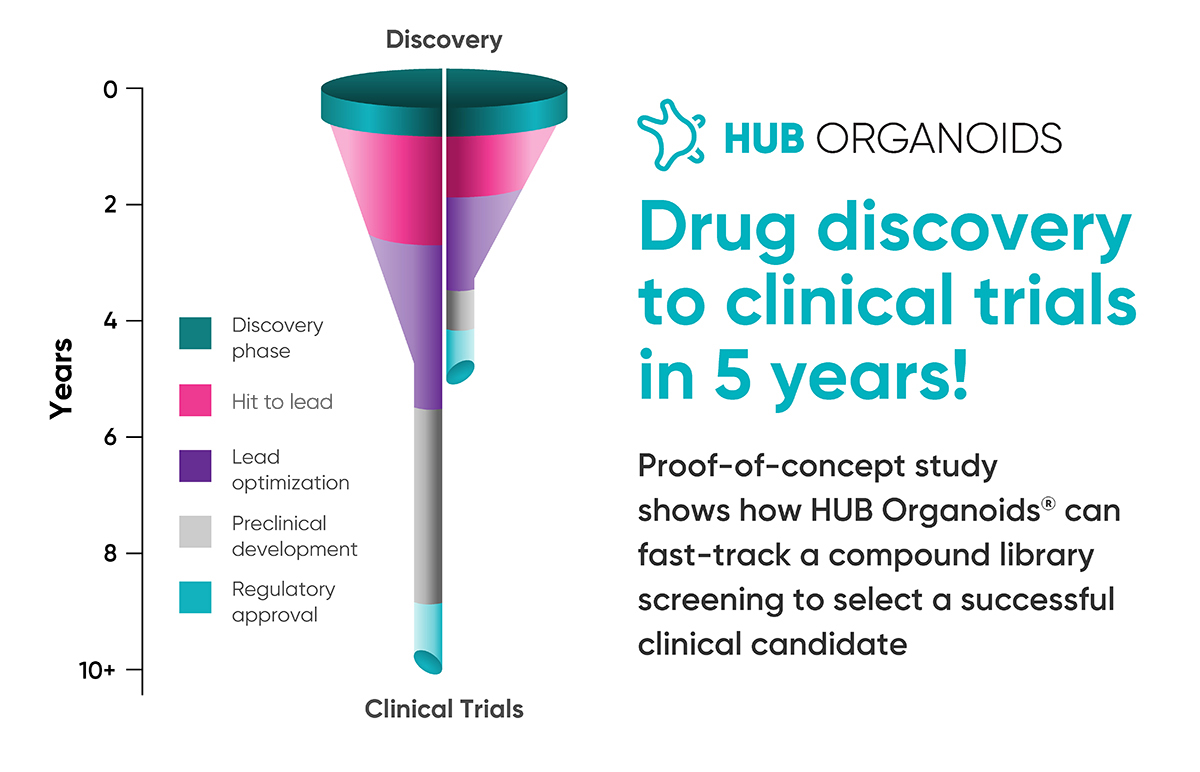
First clinical candidate developed using HUB Organoids makes it to the clinical trials within 5 years | HUB Organoids
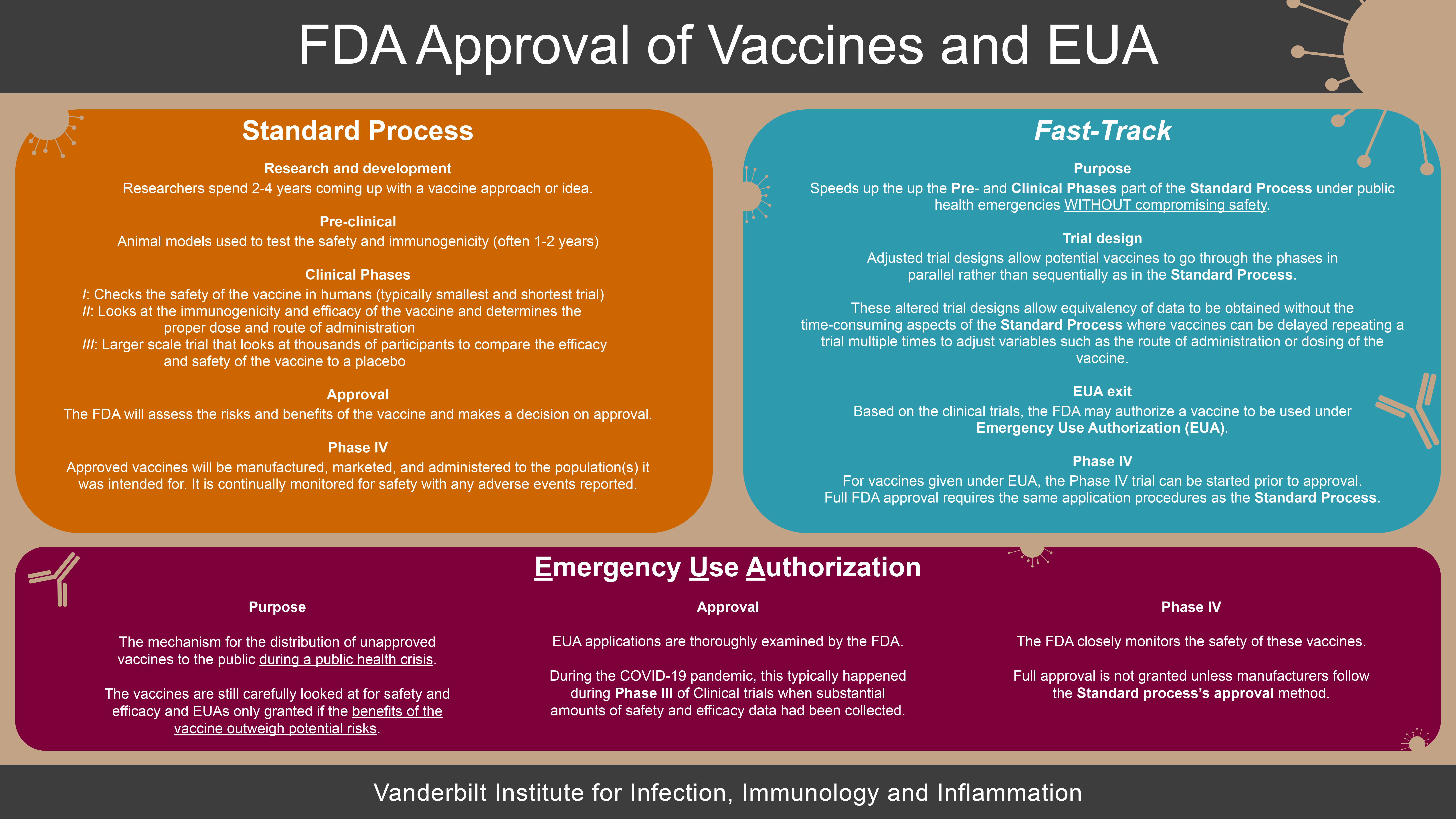
FDA Approval of Vaccines and EUA Infographic | Vanderbilt Institute for Infection, Immunology and Inflammation