Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials - The Lancet Oncology

Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials - The Lancet Oncology
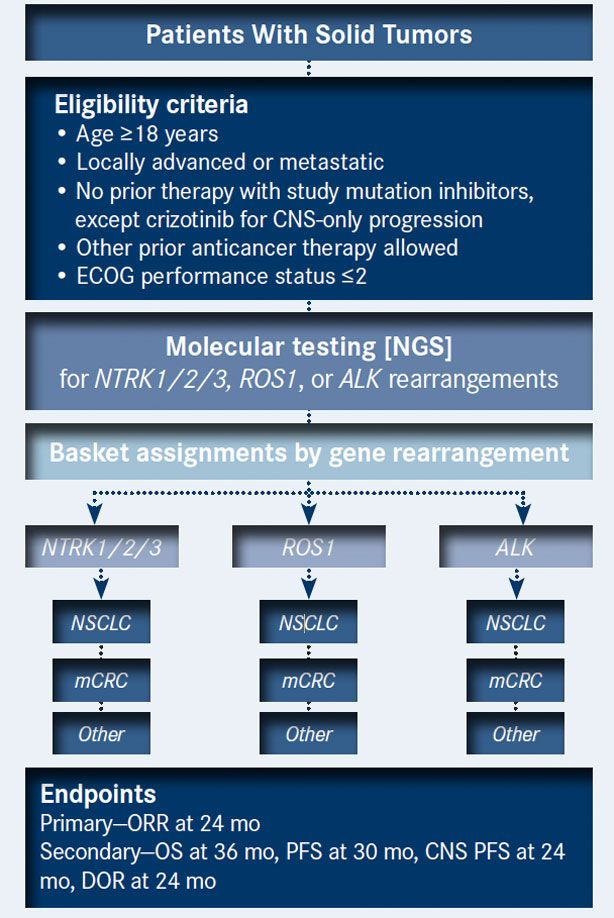
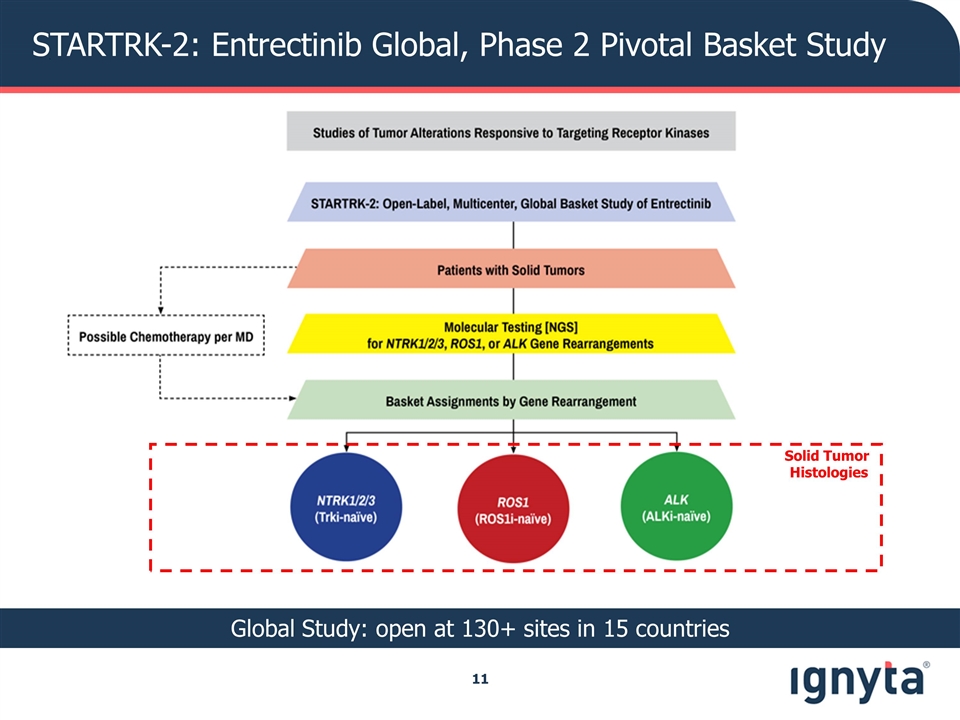
STARTRK2 Clinical Trial: A Basket Study of Entrectinib for the Treatment of Solid Tumors with Specific Gene Rearrangements

Patient-reported outcomes from STARTRK-2: a global phase II basket study of entrectinib for ROS1 fusion-positive non-small-cell lung cancer and NTRK fusion-positive solid tumours

Are you searching for clinical trials? myTomorrows can help. In our latest video, we explain what a Treatment Search Report is and how myTomorrows... | By myTomorrows | Facebook
Entrectinib in NTRK Fusion-Positive Gastrointestinal Cancers: Integrated Analysis of Patients Enrolled in Three Trials (ALKA-37

Long-Term Efficacy and Safety of Entrectinib in ROS1 Fusion–Positive NSCLC - JTO Clinical and Research Reports

Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population | npj Precision Oncology
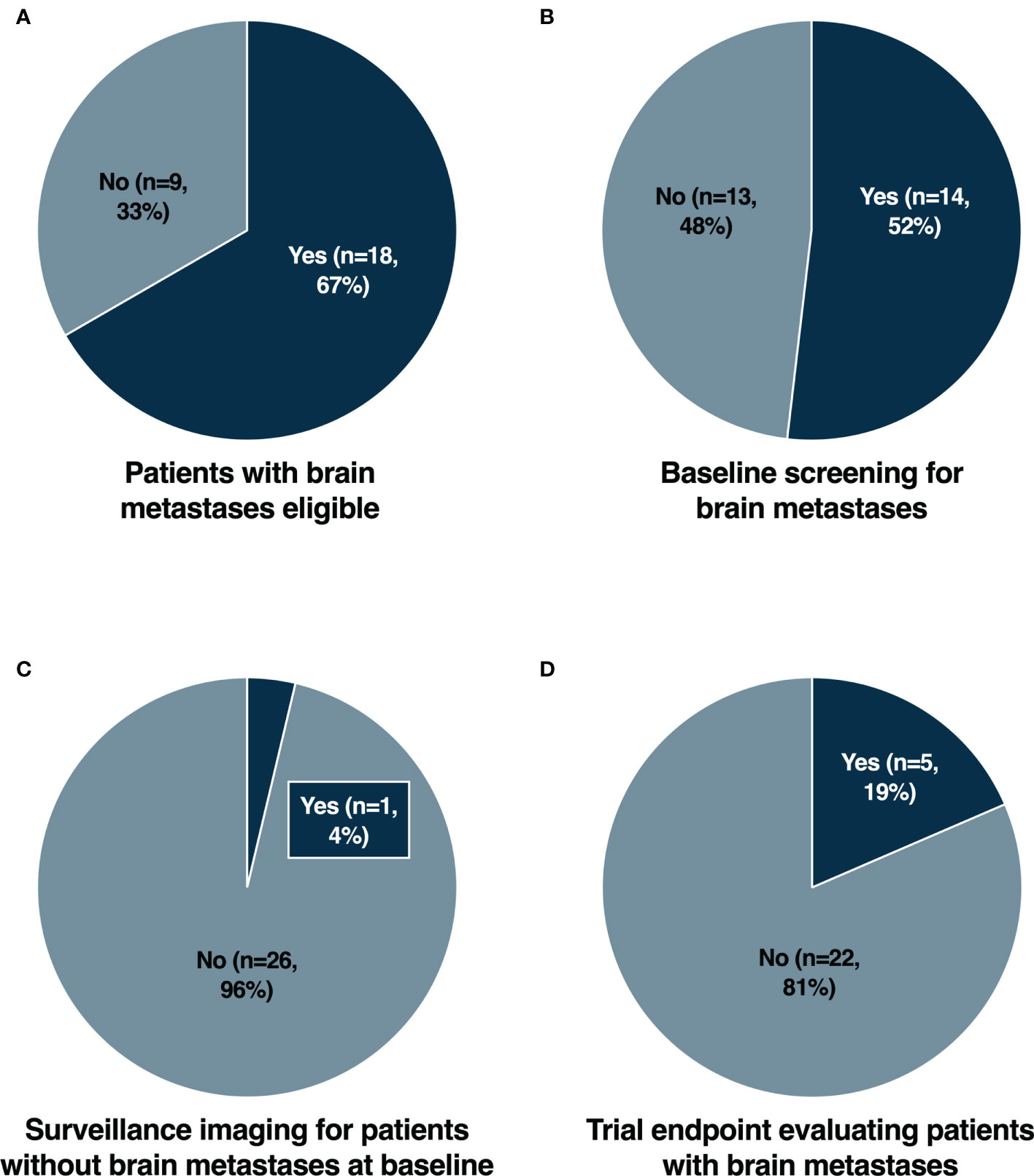
Frontiers | Clinical Trial Eligibility Criteria and Recently Approved Cancer Therapies for Patients With Brain Metastases

Patient-reported outcomes from STARTRK-2: a global phase II basket study of entrectinib for ROS1 fusion-positive non-small-cell lung cancer and NTRK fusion-positive solid tumours - ESMO Open